 Indexed in Index Medicus and Medline
Indexed in Index Medicus and Medline
HOW I DO IT
Adjunctive use of Narrow Band Imaging during transurethral resection/vaporization
of bladder tumors to aid In identifying mucosal and sub-mucosal hypervascularity
Gregory J. Diorio, DO,1 Daniel J. Canter, MD2,3
1Einstein Healthcare Network, Philadelphia, Pennsylvania, USA
2Department of Urology, Einstein Health Network/Urologic Institute of Southeastern Pennsylvania, Philadelphia, Pennsylvania, USA
3Fox Chase Cancer Center, Philadelphia, Pennsylvania, USA
DIORIO GJ, CANTER DJ. Adjunctive use of Narrow Band Imaging during transurethral resection/vaporization of bladder tumors to aid In identifying mucosal and sub-mucosal hypervascularity. Can J Urol
2015;22(2):7763-7766.
For patients with non-muscle invasive bladder cancer, cystoscopy and transurethral resection/vaporization of the bladder tumor plays an integral role in the treatment of a given patient’s bladder cancer. Although considered the current gold standard for tumor detection, traditional or white light cystoscopy has been shown to have its limitations visualizing both small papillary tumors and/or carcinoma in-situ. Current efforts have been directed to closing this gap with data demonstrating that by identifying these previously missed lesions, tumor recurrence and progression rates are reduced, thereby improving patient outcomes.
Narrow Band Imaging, which can be used during cystoscopy and transurethral resection/vaporization of bladder tumors, can aid in visualizing mucosal and sub-mucosal hypervascularity--a probable surrogate for malignant lesions--potentially visualizing the boundaries of lesions that may have been missed during white light cystoscopy alone. This technique may produce equivalent visual markers with fewer logistical hurdles than currently available methods.
In this article, we detail our technique for the adjunctive use of Narrow Band Imaging during cystoscopy and transurethral resection/vaporization of bladder tumors to aid in visualizing mucosal and sub-mucosal hypervascularity. Although not yet readily adopted, Narrow Band Imaging may be a practical and easy to use adjunct to existing methods in visualizing occult bladder lesions.
Key Words: bladder cancer, transurethral resection/vaporization of a bladder tumor, Narrow Band Imaging
- Introduction
- Methods
- Preprocedure instructions
- The procedure
- Post-procedure instructions
- Discussion
- Conclusions
- References
In 2015, there will be approximately 74,000 new cases and just over 16,000 deaths in the United States due to bladder cancer.1 According to the Bladder Cancer Advocacy Network, there are approximately 500,000 people living with bladder cancer in the United States. For patients with non-muscle invasive bladder cancer (NMIBC), cystoscopy with transurethral resection/vaporization of the bladder tumor (TURBT/TUVBT) is an integral part of a given patient’s care.
Although this approach is the current gold standard, white light cystoscopy (WLI) will fail to identify a significant number of papillary tumors or carcinoma in-situ (CIS).2 Recent technology has been introduced to visualize the boundaries of occult lesions using either photodynamic therapy (PDD) or Narrow Band Imaging (NBI). Both techniques have been shown to improve visualization of malignant lesions compared to WLI.3,4
NBI is an image-enhancement technique that augments the contrast between the bladder mucosa and microvascular structures.5 In so doing, NBI potentially will enhance both mucosal and submucosal hypervascularity. Although a recent meta-analysis affirmed the promise of NBI3 when used in conjunction with WLI, this technique requires prospective, randomized validation to confirm oncologic equivalency and safety. In this report, we detail our technique for the adjunctive use of NBI to aid in identifying hypervascularity during cystoscopy and TURBT/TUVBT.
Prior to proceeding with TURBT/TUVBT, appropriate upper tract imaging (CT urogram, MR urogram, IVP, or renal ultrasound) should be obtained to ensure that a concomitant upper tract urothelial tumor is not present. This imaging is important for patients with newly diagnosed bladder cancer, however it may not be needed in patients with a history of low-grade disease.
More recently, urologists have begun to readily adopt bipolar energy for transurethral resections. The advantages of bipolar energy using saline irrigation has been shown with prostatic tissue resection, resulting in less incidence of TUR syndrome and a reduced need for blood transfusions.6 In the past year, authors have published on the safety of bipolar bladder tumor resection using a PK Button electrode, citing the electrode’s ability to access tumors (dome and anterior wall) that traditionally are harder to resect with the loop electrocautery, its ability to vaporize in all directions, and the improved visualization afforded by the vaporization technique.7 All of these factors have resulted in a safer procedure with fewer complications.8 In addition to these safety advancements, there is still a need for improved visualization of occult bladder lesions that will result in improved oncologic outcomes. NBI may be another conjunctive option to offer this advantage in its ability to highlight hypervascularity.
1) In the past, it has been recommended that anticoagulants/antiplatelet agents should be stopped 5-7 days prior to TURBT/TUVBT. However, given the recent concern for acute stent thrombosis, especially in patients with drug-eluting stents, and our experience demonstrating minimal blood loss during TURBT/TUVBT with the Button electrode, we routinely continue patients on their anticoagulants/antiplatelet therapies.
2) A negative urine culture is obtained preoperatively.
1) Intravenous antibiotics are given 1 hour prior to instrumentation in compliance with surgical quality metrics.
2) After the induction of general or spinal anesthesia, the patient is positioned and prepared in the dorsal lithotomy position as one would for any transurethral resection. Paralysis is usually not needed due to the reduced transmission of electrical current with the Button electrode toward the obturator nerve.
3) Complete cystoscopy is then performed in the standard fashion using WLI to re-identify the primary tumor as well as to assess for tumor multifocality, Figure 1a. This assessment is sometimes more easily done in the operating room with the patient under anesthesia. Once cystoscopy is completed with WLI, we then switch to NBI by pressing the button on the camera cord. A repeat thorough cystoscopy is then done, specifically looking for areas of hypervascularity in the mucosa, Figure 1b. These areas most likely represent occult bladder lesions. These occult lesions will be biopsied. Also, we use NBI to assess the peri-tumor vascularity to try and determine the demarcation between hyper vascular tissue and normal appearing mucosa and sub-mucosa, Figure 2. Our goal is to resect/vaporize all hypervascular tissue to a margin of normal appearing bladder mucosa and sub-mucosa.
4) Once the WLI and NBI assessments are complete any identified occult lesions will be biopsied, and the biopsy site will be fulgurated. The main tumor is then resected/vaporized as has been previously described.7
a) Briefly, the rigid biopsy forceps are used to take multiple biopsies of the exophytic portions of the tumor to provide tumor grade information.
b) The bladder tumor is then vaporized using the PK Button electrode. Vaporization continues until the tumor base is reached. At this point, the operating surgeon can either take deep biopsies (usually three to four) of the tumor bed using the rigid biopsy forceps or switch to the bipolar loop electrocautery to resect the tumor bed.
c) Once all the specimens have been removed, hemostasis of all sites is confirmed.
5) During the vaporization, we routinely toggle back and forth between WLI and NBI to ensure we are vaporizing the whole tumor with a margin of normal appearing tissue. We confirm this prior to completing the procedure.
Post-procedure instructionsTop
1) To date, we have not routinely been placing a Foley catheter at the conclusion of the procedure; however, this decision is at the discretion of the surgeon.
2) The patient is discharged to home once all the post-surgical criteria are met.
The treatment goals of patients with NMIBC are to minimize treatment-related complications and morbidities and to prevent disease recurrence/progression. The use of bipolar energy has been affirmed as a safer energy source for prostatic resections because the surgeon can use saline irrigation.6 With the recent introduction of the PK Button electrode, the safety profile of transurethral resections/vaporizations of the prostate9 and bladder tumors7,8 has been further enhanced. Technologies, such as NBI, potentially can offer a novel method to visualize mucosal hypervascularity, which may represent malignant bladder lesions. If identified and treated completely at an earlier stage, it would be a reasonable hypothesis that this event would translate into improved recurrence-free and bladder-cancer-specific survivals.
To date, there has been notable experience with improved tumor detection and recurrence-free survival with photodynamic therapy. In a recent report of over 500 patients updating outcomes between patients undergoing only WLI versus WLI and PDD, the authors found that the median time to recurrence was 9.4 months compared to 16.4 months for patients also undergoing fluorescence cystoscopy.4 This time to recurrence was significantly better in the fluorescence cystoscopy group (p = 0.04).4 The median follow up for both groups of patients was just over 4 years.
Furthermore, a recent meta-analysis re-examined all the available raw data of studies using PDD to improve detection of bladder lesions. In this paper, the authors identified nine candidate studies that included 2,212 patients. The results of this meta-analysis demonstrated that PDD was almost five times more likely to identify bladder tumors across all subgroups (HR = 4.898, 95% CI = 1.937-12.390; p < 0.001),10 and, importantly, overall recurrence rates favored the PDD group (34.5% versus 45.4%) with an overall relative risk reduction of 0.761 (p = 0.006).10
Despite these findings, there are logistical challenges associated with PDD. First, the medication must be administered at least 1 hour prior to cystoscopy and allowed to dwell. Second, the ability to perform PDD does require a capital investment in the light source and cystoscopic telescopes needed to visualize the bladder mucosa during blue-light cystoscopy. In contrast, the technology with NBI is built into the existing high-definition cystoscopes and requires no further capital outlay. Furthermore, the optical imaging can be performed during cystoscopy by simply pressing a button on the camera cord, requiring no special pre-procedure catheterization and drug administration. In addition to these less burdensome logistical concerns, the initial clinical experience with NBI has been very promising. In a systematic review and meta-analysis of over 1000 patients where NBI was used in enhancing mucosal hypervascularity, an additional 24% more bladder cancer tumors were visualized using NBI.3 Furthermore, 28% more carcinoma in situ tumors were discovered using NBI versus WLI.3 Importantly, there was not a significant difference in false positives between the two groups. This technology appears to have a significant potential to aid in enhancing mucosal hypervascularity that could translate into improved visualization of lesion boundaries. Clearly, further clinical studies are warranted.
Narrow Band Imaging potentially offers an easier to use technology to identify mucosal hypervascularity which should translate into the visualization of occult bladder lesions. Finding these bladder lesions that typically would not be seen on white-light cystoscopy should result in improved bladder-cancer related survivals. Further prospective clinical study is warranted to validate this technology in the treatment/detection paradigm of patients with non-muscle invasive bladder cancer.
Accepted for publication March 2015
Address correspondence to Dr. Daniel J. Canter, Department of Urology, Einstein Health Network, Urologic Institute of Southeastern Pennsylvania, 50 E. Township Line Road, Elkins Park, PA 19027 USA
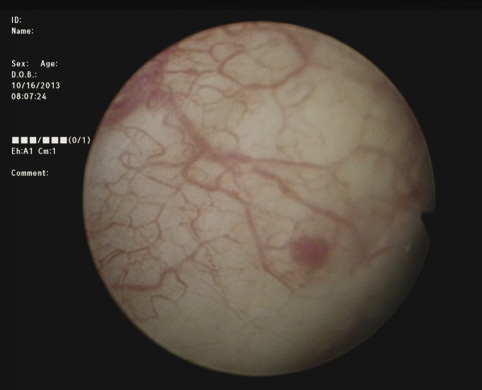
Figure 1a. White light cystoscopy demonstrating flat bladder lesion.
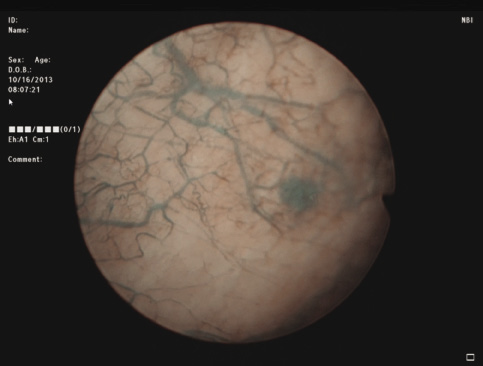
Figure 1b. Cystoscopy of same lesion adjunctively using Narrow Band Imaging, highlighting tumor and surrounding vascularity.
Diorio and Canter
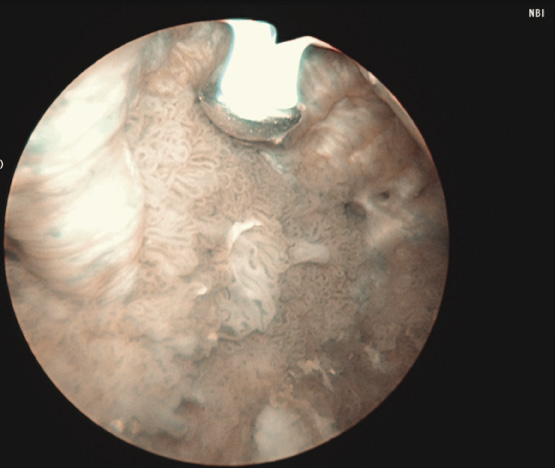
Figure 2. Adjunctive use of Narrow Band Imaging during bladder tumor resection using the Button Bipolar electrode. Narrow Band Imaging helps to delineate vascularity associated with tumor and vascularity of normal surrounding urothelium.
1. American Cancer Society: Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015.
2. Jichlinski P, Leisinger HJ. Fluorescence cystoscopy in the management of bladder cancer: a help for the urologist! Urol Int
2005;74(2):97-101.
3. Li K, Lin T, Fan X, Duan Y, Huang J. Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Urol 2013;20(6):602-609.
4. Grossman HB, Stenzl A, Fradet Y et al. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol 2012;188(1):58-62.
5. Herr HH. Narrow band imaging cystoscopy. Urol Oncol 2011;29(4):353-357.
6. McVary KT, Roehrborn CG, Avins AL et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185(5):1793-1803.
7. Canter DJ, Ogan K, Master VA. Initial North American experience with the use of the Olympus Button Electrode for vaporization of bladder tumors. Can J Urol 2012;19(2):6211-6216.
8. Geavlete B, Multescu R, Georgescu D, Jecu M, Dragutescu M, Geavlete P. Innovative technique in nonmuscle invasive bladder cancer-bipolar plasma vaporization. Urology 2011;77(4):849-854.
9. Reich O, Schlenker B, Gratzke C et al. Plasma vaporisation of the prostate: initial clinical results. Eur Urol 2010;57(4):693-697.
10. Burger M, Grossman HB, Droller M et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol 2013;64(5):846-854.

