 Indexed in Index Medicus and Medline
Indexed in Index Medicus and Medline
Free Articles
HOWIDOIT
How I do it: percutaneous radiofrequency ablation (RFA)
Igor Sorokin, MD1 Murthy Chamarthy, MD,2 Jeffrey A. Cadeddu, MD1,2
1Department of Urology, UT Southwestern Medical Center, Dallas, Texas, USA
2Department of Radiology, UT Southwestern Medical Center, Dallas, Texas, USA
SOROKIN I, CHAMARTHY M, CADEDDU JA. How I do it: percutaneous radiofrequency ablation (RFA). Can J Urol 2017;24(1):8679-8683.
Percutaneous radiofrequency ablation has seen increased utilization secondary to the rising incidence of renal cell carcinoma. This has been shown to be an effective and durable treatment especially in the elderly comorbid patient. In this article, we describe our technique and factors related to successful outcomes.
Key Words: percutaneous radiofrequency ablation, RFA, thermal ablation, renal mass, kidney cancer, renal cell carcinoma
Introduction
The rising incidence of renal cell carcinoma (RCC) in the last several years has led to increased utilization of thermal ablation (TA) for small renal masses (SRM).1 A recent systematic review on management of localized renal cancer concluded that overal survival rates are similar for TA, partial nephrectomy (PN), and radical nephrectomy (RN) and are primarily determined by patient age and comorbidity.2 The two most popular methods of TA are cryoablation and radiofrequency ablation (RFA) performed either percutaneously or laparoscopically. These procedures are ideally suited for small, clinically localized, enhancing renal mass (≤ 4 cm) in an elderly comorbid patient. However, recent literature has shown that survival outcomes are favorable in healthy adults undergoing RFA for T1a renal tumors.3 With the increased incidence of RCC and subsequent increased utilization of TA, it is important for the urologist treating SRM to be versed in the technique. We prefer to use percutaneous RFA at our instituition and describe our technique and tips and tricks learned over our 15 year experience.
Surgical technique
Principles of RFA
It is critical for urologists to understand the principles of RFA prior to performing an ablation. The concept is simple and involves creating an electrical circuit where monopolar alternating current produced by the generator flows through the patient between the radiofrequency (RF) probe and the grounding pads. This alternating current results in ionic agitation and heat-producing molecular friction around the probe with resultant thermal damage. When temperatures reach above 60°C, direct cytotoxic effects occur on cellular components, as well as secondary ischemic injury from microvascular and arteriolar occlusion.4
Patient selection and preparation preoperatively
Computed tomography (CT) scanning using 3 mm axial cuts with and without intravenous contrast (IV) is important to delineate the tumor, plan your approach, and decide whether focal ablation is safe and appropriate. The size of the tumor is important as survival outcomes and recurrences for cT1a lesions are better than cT1b after RFA.5 Even when cT1a are sub-stratified by size, the smaller the lesion, the better the results.6,7 It is reasonable to ablate larger lesions in comorbid patients who cannot undergo PN but it may require multiple probe deployments and increased operative time. Location of the tumor is also important as anterior and medial tumors are better approached laparoscopically, while posterior and lateral lesions are approached percutaneously. Centrally located tumors are poor candidates for TA because of the "heat-sink" phenomenon where high regional vascular flow may prevent attainment of lethal temperatures and interfere with thermally induced coagulation. Patients found to have multifocal and/or bilateral tumors are good candidates for percutaneous RFA as ablations can be performed at multiple sites in a staged approach. Patients with solitary kidneys may fair better after RFA, as renal function preservation has been shown to be greater with RFA compared to PN.8
Recognizing vital adjacent structures in proximity to the tumor and probe trajectory tract are extremely important. The presence of bowel or ureter within 1 cm of the ablation zone is a relative contraindication to RFA as thermal injury can occur. Further contraindications include obstruction of the probe tract by intervening liver, spleen, or lung. Patients whose tumors lie close to their body-wall musculature may experience greater pain in the perioperative period.9
Preoperative laboratory testing includes blood coagulation screening, urine culture, serum electrolytes, liver function tests, and serum creatinine. Patients should discontinue anticoagulation 5-7 days prior to the procedure.
A diagnostic biopsy should be performed prior to or at the time of ablation.10 This should be done with a coaxial technique to minimize the risk of complications and seeding. Furthermore, this helps in refining post-ablation follow up.
Patient positioning and anesthesia
General anesthesia is induced while the patient is supine on the stretcher. In our experience, controlling the respirations while under general anesthesia allows for more accurate positioning of the probe to achieve a complete ablation.11 After induction, a foley catheter is placed to monitor for hematuria during the procedure and the patient is then positioned prone on two large gel rolls. Pressure points are also padded with foam. Grounding pads are placed equidistant below the gluteal crease on the back of each leg. Large straps are then used to secure the patient in the CT-scanner bed. The area over Petit's triangle is left exposed and then prepped sterilely with betadine.
Prone scan and procedure planning
In an ideal case, there are three CT scans performed. The first CT scan is performed in the prone position using IV contrast (50% of standard dose) to plan the procedure. It is important to pay attention to surrounding structures as abdominal contents could have shifted compared to a supine CT scan. Patients with renal insufficiency can be challenging as contrast is usually avoided. Therefore, one must rely on cortical changes in the non-contrast phase to delineate the lesion, Figure 1. The trajectory of the probe is planned and measured. It is of high importance to be able to insert the probe as straight as possible (perpendicular to the skin) toward the mass. If a mass is quite medial as shown in Figure 1, it is best to plan the placement of the probe slightly lateral off the paraspinal muscles to avoid pain.
Probe insertion
After adequate planning, 1% lidocaine is injected into the tract and an 18 gauge needle is left hubbed from the injection to determine whether it is in the vicinity of the mass. If properly positioned, this needle is exchanged for the RF probe after making a small incision. We prefer to use a 15-gauge RITA StarBurst probe (RITA Medical Systems, Mountain View, CA, USA) as the tine configuration permits visual confirmation that the entire tumor is enveloped. Figure 2 shows an example of how the tines appear deployed outside of the body and by CT scan. Under CT fluoroscopy guidance the probe is always inserted during a breath hold at end-expiration. The probe is advanced to puncture the tumor and then the tines are deployed rapidly. If inserted too slowly, the tines may not fully deploy. They should extend slightly past the tumor margin to ablate 0.5 cm beyond the mass. Near-perpendicular insertion of the probe on the mass is critical as it allows the greatest energy to be deposited at this deep margin where the region of highest blood flow is expected.
Probe confirmation
The 2nd CT scan is performed without IV contrast to visualize the trajectory of the probe and ensure that a normal rim of parenchyma is ablated at the deepest margin. Adjustments to the probe should be made at this point. When dealing with larger tumors for example, the tine array may not envelope the entire extent of the tumor and multiple ablations may be required. The probe should cover one aspect of the tumor and the unablated portion can be measured on the CT scan. As the ablated zone from one tine will extend 5 mm, this can be used to plan repositioning, Figure 3.
Ablation
Our ablation technique involves the use of a temperature-based generator (RITA model 1500X, RITA Medical Systems, Mountain View, CA, USA). The RF probe is connected to the generator by a heavy black cord. A trick to prevent dislodgement of the probe is to secure it to the CT scanner. The target temperature is set to 105°C and maximum power to 150 Watts. Time of ablation is dependent on tumor size: 5 minutes for < 2 cm, 7 minutes for < 3 cm, 8 minutes for < 4 cm. Once the ablation is begun, the generator increases power slowly so that temperature is raised slowly to prevent desiccation and char. The ablation countdown time only begins once target temperature is reached. The probe itself has nine tines of which five are connected to thermocouples that constantly monitor temperature. At the end of the cycle, a cool down cycle is automatically performed with the goal that tissue temperature is maintained at least above 65°C for 30 seconds. As a reference, tissue that maintains this temperature during this cycle confirms that cell death has occurred in the ablation zone. Our institution's protocol is to repeat this ablation for a second time using the same settings, tine deployment, time, and an additional cool down cycle.
A track ablation is performed to prevent bleeding and the risk of tumor seeding. This is performed by pressing the "track ablation" mode on the generator, pulling the tines back on the probe and then withdrawing the probe slowly (usually 1 cm length). The goal is to maintain temperatures above 75°C. If temperature is below this, the probe should be withdrawn at a slower speed.
Immediate post-ablation CT scan and follow up
The final CT scan is performed immediately after ablation with IV contrast (50% of standard dose) to confirm successful ablation. A rim of demarcation seen around the ablation zone signifies a successful treatment. The immediate post-ablation scan is also important to identify complications such as bleeding. Figure 4a represents the tissue changes expected to be seen immediately after ablation.
The American Urological Association guidelines10 recommend obtaining cross sectional imaging with either CT or MRI 3-6 months after ablation and this serves as a baseline to compare future scans. Thereafter, annual abdominal scans are recommended for 5 years. Figure 4b shows the typical "halo" scar expected to be seen several months after ablation. Absence of enhancement on CT or MRI has been shown histologically to be consistent with cancer kill.12 Thus, the urologist should be suspicious of treatment failure or local recurrence when imaging reveals a visually enlarging neoplasm or new nodularity in the ablation zone that enhances with contrast.
Discussion
RFA outcomes are typically compared to PN and cryoablation. A recent systematic review showed excellent cancer-specific survival between thermal ablation and partial nephrectomy, which was primarily determined by tumor size, rather than approach.2 Overall survival was better for partial nephrectomy in account for the selection bias. Although low strength of evidence, renal function outcomes were similar for thermal ablation and PN. Complication rates are lower for both cryoablation and RFA compared to PN.
We reported our oncologic results treating 243 renal masses over 7.5 years (years 2001-2008) using either laparoscopic or percutaneous RFA. The overall 5-year recurrence free survival rate was 93% (90% in those that had biopsy proven RCC).13 Analyzing only solitary T1a lesions, we demonstrated similar 5 year overall survival (97% versus 100%) and disease-free survival (89% versus 89%) for laparascopic/percutaneous RFA when compared to open/laparoscopic PN, respectively.14 RFA outcomes with larger lesions are not as good and we have reported a discrepancy in 5 year disease free survival (95% versus 79%, p = 0.001)
for tumors < 3 cm to those ≥ 3 cm, respectively.7 We have also reported on RFA outcomes in healthy adults with T1a lesions showing 5 and 10 year overall survival of 96% and 91%, respectively and 5 and 10 year recurrence-free survival of 94%.3
Our published results have included both percutaneous and laparoscopic techniques for RFA. We have since abandoned the laparoscopic approach and perform RFA solely by the percutaneous technique described. If a patient is healthy enough to undergo laparoscopy, we feel robotic-assisted laparoscopic PN is a better treatment modality. Furthermore, active surveillance has been proven to be a good option2 and if a mass can only be reached laparoscopically, this is a good alternative in the comorbid patient.
Our success with RFA is likely attributed to multiple factors. Patient selection is certainly key and smaller T1a lesions are more favorable with this approach. The deployed tine array of the RITA StarBurst probe also enhances confidence that the ablation zone encompasses the tumor. It is important to be very meticulous about the ablation zone coverage and if not satisfied, the probe should be repositioned until "perfect" coverage is achieved. Our institution policy is also to use a double ablation method by repeating the RF cycle using the same settings and time. We believe all these factors are important in achieving successful results with RFA.
Conclusions
Percutaneous RFA is a durable option for treating small renal neoplasms. It is associated with recurrence-free survival comparable to PN and cryoablation. Furthermore, the low complication rates and minimal hospital length of stay is beneficial for treating elderly comorbid patients.
Accepted for publication December 2016
Address correspondence to Dr. Jeffrey A. Cadeddu, Department of Urology, UT Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390 USA
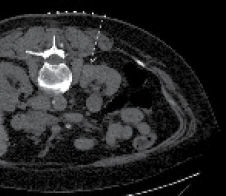
Figure 1. Non-contrast planning image of patient with renal insufficiency. Notice the cortical and contour changes that help delineate the mass. Typically, it is best to come down as perpendicular on the mass as possible. With lesions located very medial, it is best to come slightly lateral off the paraspinal muscles when planning the trajectory of the probe (dashed line) to avoid pain.
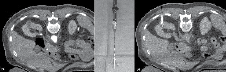
Figure 2. A) Before probe placement a CT scan is performed with IV contrast showing a 2.4 cm partially exophytic posterior ride sided tumor. B) The RITA StarBurst probe is shown with tines deployed. C) The configuration of the probe permits visual confirmation of coverage of the mass. Notice the ideal straight trajectory of the probe to the mass.
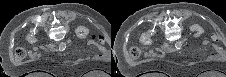
Figure 3. A) A bilobed shaped 4 cm right sided tumor is shown. The probe was initially deployed on the inferior aspect of the tumor with tines seen extending into a rim of normal parenchyma. The first ablation was then performed. B) After completion of the first ablation, the probe was repositioned to address the superior aspect of the tumor. After proper probe placement, the second ablation cycle commenced.
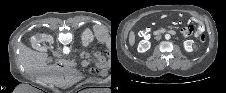
Figure 4. A) Immediate post-ablation scan with IV contrast showing a rim of demarcation around the ablation zone. This represents a successful treatment. B) The typical "halo" scar expected to be seen several months after ablation. This particular example is of a left renal mass 12 months after RFA.
References
1. Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol 2011;186(2):394-399.
2. Pierorazio PM, Johnson MH, Patel HD et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol 2016;196(4):989-999.
3. Ma Y, Bedir S, Cadeddu JA, Gahan JC. Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int 2014;113(1):51-55.
4. Corwin TS, Lindberg G, Traxer O et al. Laparoscopic radiofrequency thermal ablation of renal tissue with and without hilar occlusion.
J Urol 2001;166(1):281-284.
5. Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63(3):486-492.
6. Moskowitz D, Chang J, Ziogas A, Anton-Culver H, Clayman RV. Treatment for T1a renal cancer substratified by size: "less is more". J Urol 2016;196(4):1000-1007.
7. Best SL, Park SK, Youssef RF et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol 2012;187(4):1183-1189.
8. Raman JD, Raj GV, Lucas SM et al. Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int 2010;105(4):496-500.
9. Baker M, Anderson JK, Jaffer O, Trimmer C, Cadeddu JA. Pain after percutaneous radiofrequency ablation of renal tumors.
J Endourol 2007;21(6):606-609.
10. Donat SM, Diaz M, Bishoff JT et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 2013;190(2):407-416.
11. Gupta A, Raman JD, Leveillee RJ et al. General anesthesia and contrast-enhanced computed tomography to optimize renal percutaneous radiofrequency ablation: multi-institutional intermediate-term results. J Endourol 2009;23(7):1099-1105.
12. Raman JD, Stern JM, Zeltser I, Kabbani W, Cadeddu JA. Absence of viable renal carcinoma in biopsies performed more than 1 year following radio frequency ablation confirms reliability of axial imaging. J Urol 2008;179(6):2142-2145.
13. Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer 2010;116(13):3135-3142.
14. Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol
2012;61(6):1156-1161.
8683

